Top 10 Benefits of Using a Fleet Management System
Managing fleets without technology is like trying to navigate a modern city with a paper map from the 1950s — you might reach your destination eventually, but expect delays, confusion, and plenty of wrong turns along the way.
Every wrong turn, delay, or detour creates a ripple effect — increasing fuel costs, extending delivery times, and ultimately hurting customer satisfaction.
Imagine you run a delivery business trying to meet tight deadlines during the Diwali rush. Your driver, unfamiliar with the city, relies on a paper map to find delivery locations. Inevitably, he’ll struggle with directions, lose valuable time, consume more fuel, and risk missing delivery deadlines — leaving customers unhappy.
Meanwhile, as the business owner, you’ll have no real-time visibility into where your drivers are, which deliveries are complete, or whether your team is safe. The only way to stay updated would be through constant phone calls or messages — a tedious task for you and a constant distraction for your drivers. This is where a Fleet Management System (FMS) transforms the game.
It’s a powerful digital solution that brings precision, efficiency, and complete control to fleet operations. As fleets grow and customer expectations rise, managing logistics through memory, phone calls, or spreadsheets is no longer enough.
Modern businesses need a system that lets them track, plan, and optimize every fleet activity — all at their fingertips.
How Were Fleets Managed Earlier?
Before the rise of digital fleet management software, fleets were managed in a much more traditional and manual way. Fleet owners largely depended on what the drivers reported and had little choice but to trust that information.
Tasks such as finding delivery locations, planning routes, scheduling maintenance, and organizing daily operations were time-consuming and prone to mistakes. Naturally, this traditional approach came with several limitations.
Paper Logs and Spreadsheets:
Earlier managers’ stored data related to vehicle maintenance, fuel usage, and driver activities on paper or basic spreadsheets. However, updating changes, verifying the data, and retrieving this information was a slow and error-prone procedure.
Phone Calls / Radio Communication:
Tracking vehicle locations or checking delivery status required constant phone calls, pagers, or radio conversations. This led to miscommunication or delayed responses.
Reactive Maintenance:
Maintenance was performed only after breakdowns or visible issues in the fleet. This resulted in unexpected downtime and higher repair expenses.
Low Visibility:
Fleet managers had little insight into vehicle locations or performance, making route optimization and cost management nearly impossible.
Compliance Challenges:
Managing documents such as licenses, insurance, and regulatory paperwork manually increased the risk of errors and non-compliance penalties.
Fuel Inefficiency:
Without tools to monitor routes or driver behavior, excessive idling, detours, and inefficient driving often led to higher fuel costs.
Long story short, manual fleet management was inefficient, reactive, and risky. Businesses found it increasingly difficult to maintain control and deliver consistent performance.
What Is Fleet Management System (FMS)?
A Fleet Management System (FMS) is software that provides real-time visibility into your fleet. It helps monitor vehicle locations, optimize routes, track fuel consumption, schedule preventive maintenance, and ensure driver safety and compliance. It serves as the “control center” of the business that brings efficiency, cost savings, and reliability to fleet operations.
Some of the industries that benefit the most:
Fleet Management software benefits those industries that relies on vehicle operations. Common sectors include:

Top 10 Benefits of Fleet Management System:
Fleet Management Systems (FMS) bring together GPS tracking, IoT integration, telematics, and analytics to simplify fleet operations and improve performance. From monitoring vehicles to optimizing costs, the fleet management software benefits businesses a lot. Here are some of them-
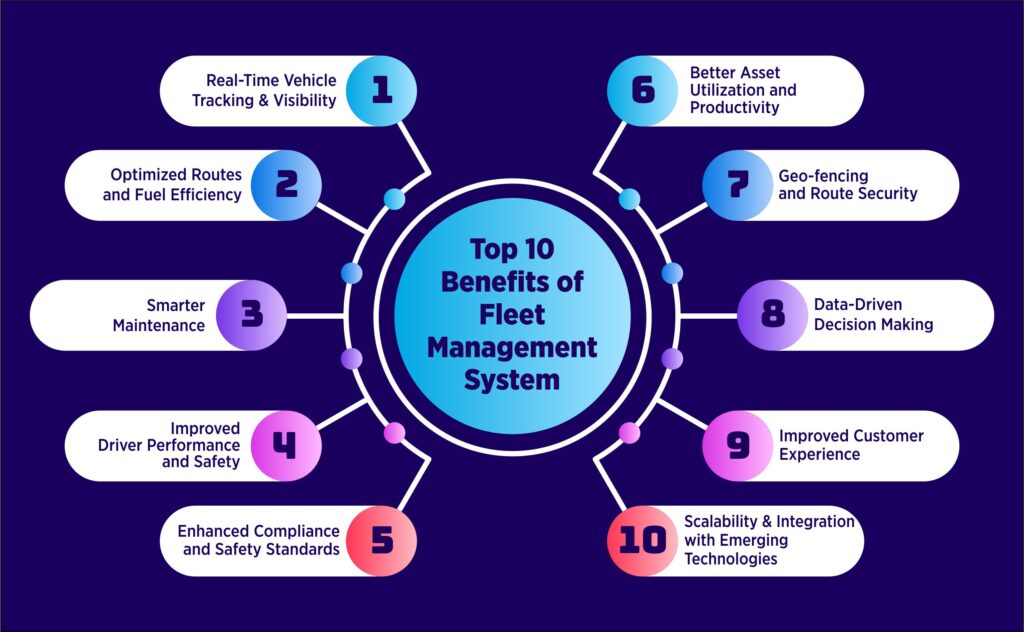
1. Real-Time Vehicle Tracking & Visibility
Real-time tracking helps business owners stay updated on the live location of every vehicle using GPS and IoT sensors. It helps prevent theft, enables quick rerouting during traffic or delays, and provides full transparency across the entire journey/trip.
2. Optimized Routes and Fuel Efficiency
FMS uses route optimization tools to suggest the most efficient paths based on live traffic, distance, and delivery schedules. This significantly reduces fuel consumption, idle time, and overall operational expenses.
3. Smarter Maintenance
FMS sends alerts for upcoming maintenance or performance anomalies. Predictive maintenance ensures issues are resolved before they cause breakdowns, extending vehicle life and minimizing downtime.
4. Improved Driver Performance and Safety
Telematics and driver behavior monitoring tools track speeding, harsh braking, idling, and route deviations. Insights from this data help promote safer, more fuel-efficient driving and reduce accident risks.
5. Enhanced Compliance and Safety Standards
Automated reminders and digital documentation ensure timely renewals of insurance, permits, and inspections. FMS also helps meet industry and government safety standards with complete audit trails and digital record-keeping.
6. Better Asset Utilization and Productivity
Monitor how and when each vehicle or asset is being used. Reduce idle time, optimize scheduling, and ensure that every asset contributes to productivity, leading to higher fleet efficiency.
7. Geo-fencing and Route Security
Fleet Management Systems allow you to create virtual geographic boundaries (geofences). Managers receive instant alerts when vehicles enter or exit designated zones—enhancing security, preventing unauthorized trips, and improving delivery tracking accuracy.
8. Data-Driven Decision Making
Every trip, delivery, and driver interaction generates valuable data. FMS turns this into actionable insights through dashboards and reports—helping managers make informed, strategic decisions for cost control and performance improvement.
9. Improved Customer Experience
Accurate ETAs, live delivery tracking, and faster responses improve reliability and customer satisfaction. Real-time updates ensure transparency and trust, especially in logistics and delivery-driven industries.
10. Scalability and Integration with Emerging Technologies
As your fleet grows, your FMS grows with you. Cloud-based platforms easily integrate with IoT devices, ERP systems, fuel cards, and telematics tools—ensuring smooth scaling and future readiness as technology advances.
Though there are many advantages of fleet management apart from this. And one such FMS that can help you manage all fleet-related activities is FleetPro by TecWork. It is an advanced fleet management software that helps eliminate fleet management, tracking, and monitoring challenges. If you want to know more about it, then click here and have a word with us.