The Benefits of Quality Management Systems: Why You Need a QMS?

Quality is a diversified term used in day-to-day life! Whether it’s the products we purchase or the services we seek, our chief expectation is excellence in the quality.
An old business saying that persists till today- “Success of any business/organization hinges on these three pivotal elements: Price, Service and Quality”. Nevertheless, the underlying truth is that businesses find it challenging to achieve all the three simultaneously.
Striking a balance is intricate, as efforts to emphasize price and service often come at the expense of quality, and a focus on service and quality may impact pricing.
One way of ensuring that all the three factors co-exist is possible by adopting a Quality Management System.
A Quality Management System is a set of standardized procedures and policies that provides a structured approach to quality management, eliminating risks and improving processes organization wide.
How Quality & Standardization Came into Existence?
The roots of the term “Quality” can be traced centuries back when the Industrial Revolution took place. Earlier, only the standards that control the processes and products were used in the form of quality management systems.
Later, as the organisations and production quantities grew, the need for best practices, to ensure quality results, was felt.
Eventually, best practices for controlling products and processes were established and documented, which turned into standard practices and termed as “Quality Management System.”
It is also believed that the need of Quality Management became increasingly important after the World War II. This was the time when, bullets and rifles used to be manufactured in separate states.
So, to ensure their effectiveness and compatibility, without sacrificing quality, armed forces started incorporating best practices into their production and performing inspection using quality techniques.
Over the time, concerning the safety of consumers, a certification was developed under the name ISO 9001. Before getting this certification, a company needs to undergo an assessment by the third-party certification authoritative body, to ensure that organization is strictly adhering to these standard processes, procedures, and activities, maintaining quality management.
This helped them ensure quality, consistency, and accuracy throughout the production.
Still wondering how does a Quality Management System benefits organizations. Read on the remaining half part of the blog to know-
Explore the Unconventional Benefits of a Quality Management System
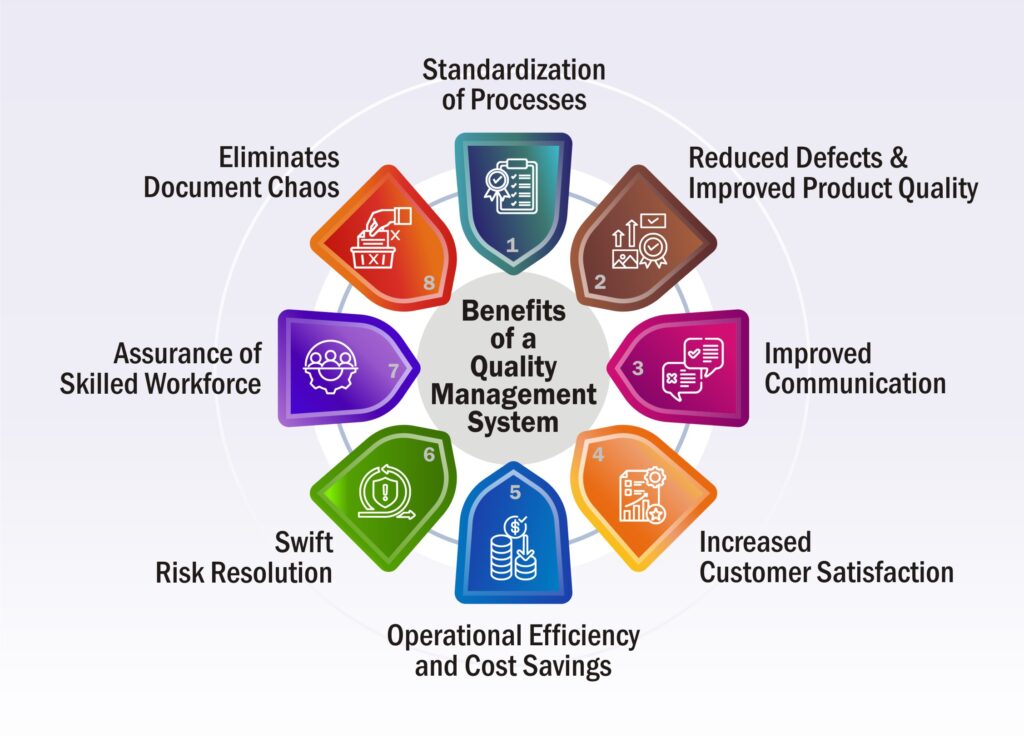
Fostering Consistency & Transparency-
A Quality Management System works justifying the phrase “Do what you documented and document what you do”, ensuring that every process is properly documented. This includes creating comprehensive documents, mentioning clear quality standards, such as Standard Operating Procedures (SOPs), work instructions, and guidelines etc.
This ultimately streamlines and standardizes the workflow, ensuring every step is executed with consistency, across the organization.
Reduced Defects & Improved Product Quality-
A Quality Management System categorizes various documents, and ensures that all the processes are being performed adhering to the set quality standards. This ultimately leads to minimizing the chances of errors and defects, improving the quality of the outcome.
Moreover, incorporating the practice of regular audits, inspections, and monitoring processes on various quality parameters, a Quality Management System helps to identify deviations (NCs), perform root cause analysis using the methods like Five Whys, Fishbone Diagram, Flow chart, Pareto chart, etc., so that corrective actions can be taken to prevent recurrence of issues.
Improved Communication-
A Quality Management System maintains a centralised repository of all the documents, including procedures and work instructions. This ensures that everyone has same information, and all the employees have a clear understanding of their roles and responsibilities, eliminating miscommunication and confusion.
Increased Customer Satisfaction-
Adopting a Quality Management System helps establish a culture of quality throughout an organization, right from top-level management to front-line employees. This ensures organizations to be customer centric, focused on fulfilling all the needs of customers.
Additionally, by offering a comprehensive solution, right from registration to resolution of complaints along with designating their source, priority, and severity, a QMS helps retain customers and increase sales.
Operational Efficiency and Cost Savings-
As discussed, since processes will be documented and standardized, workflow will be streamlined. Consequently, there will be less room for error, contributing to reduced wastage, increased cost savings, and enhanced operational efficiency.
Swift Risk Resolution-
The proactive approach of a Quality Management System to ensure that standardized processes are regularly followed across the organization helps identify, assess, and create a framework of steps to mitigate the risks related to compliance, safety, and product/service quality.
Assurance of Skilled Workforce-
A Quality Management System streamlines the complete training process right from helping identify the training requirements, maintaining record of trainees, scheduling sessions, execution, to evaluating performances. This type of comprehensive approach ensures that every employee stays updated by continually improving skills.
Additionally, it also aids organizations in assigning specific roles to specific individuals based on their results, minimizing the likelihood of errors.
Need to Switch to Quality Management Software-
However, a Quality Management System that relies on manual intervention becomes error-prone and time-consuming, resulting in inefficient operations. To avoid this, a Quality Management Software was developed.
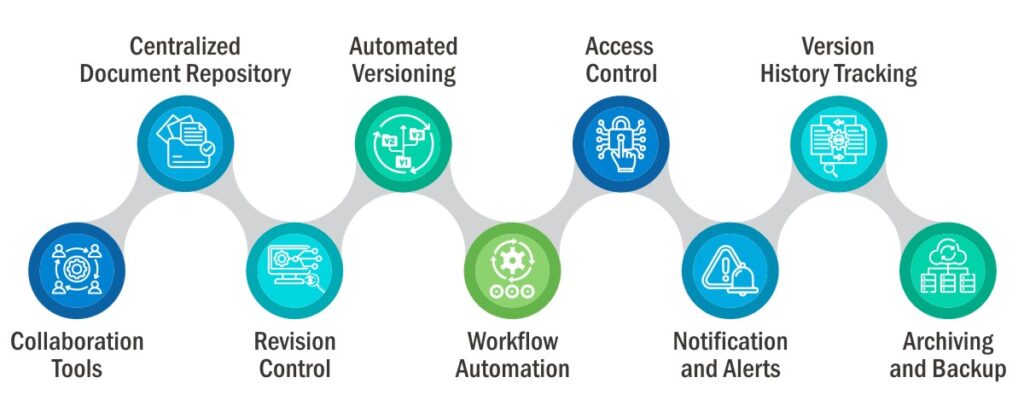
It is a technological platform that allows to perform all the quality related operations and manage business crucial documents, digitally!
This tool automates and simplifies the core quality management processes involving:
- Document management
- Risk management
- Training management
- Complaint management
- Audit management
- SOP management
- Inspection
- Non-conformance management, etc.
Final Thoughts-
Adopting a Quality Management System (QMS) brings numerous benefits, fostering a culture of quality and continuous improvement within the organization. However, these advantages remain out of reach if quality management processes rely on outdated, manual methods.
To unlock the full potential of a QMS, digitization is essential—and that’s where a robust Quality Management System Software comes into play.
The real challenge lies in selecting the ideal QMS that offers all the essential features and aligns with your organization’s needs. Enter QualityPro—a cutting-edge, technologically advanced software crafted to digitize and optimize your quality management processes.
Packed with powerful modules, this next-gen, cloud-based solution ensures your organization stays ahead in maintaining and elevating quality standards.
No matter your industry, QualityPro by TecWork transforms your quality management program, making it more efficient and impactful.
Have questions about implementation? Our team of experts is here to help. Contact us today and take the first step toward revolutionizing your quality management system!