Pharmaceutical Quality Management Software
Elevate product quality, streamline deviation and CAPA control, and accelerate batch release with an efficient pharmaceutical QMS Software.

Major Quality Management Challenges in the Pharmaceutical
Superior quality and customer satisfaction are critical aspects of pharmaceutical manufacturing. Patients rely on medicines for their well-being, expecting high-quality drug compositions that are safe, effective, and free from contamination or defects.
Quality is a key factor that builds patient trust in pharmaceutical products. Any deviation from quality, efficacy, or safety standards can lead to regulatory recalls, financial losses, and reputational damage for pharmaceutical manufacturers.
To address these challenges, companies implement robust Pharma Quality Management Software to maintain compliance, improve process efficiency, and mitigate risks.
Pharmaceutical manufacturers must proactively prevent and manage quality issues caused by non-compliance, process modifications, human errors, and regulatory changes. This is where Pharma QMS Software plays a pivotal role.
Request a Free Demo
Trusted by Industry Leaders








How Does Pharma QMS Software Help Address These Challenges?
Understanding the significance of a QMS in pharma, deploy advanced pharmaceutical quality management software like QualityPro to maintain high standards across products, processes, and services.
QualityPro is best-in-class QMS software for pharmaceutical manufacturing industry, enabling companies to:
- Develop risk-based CAPA process
- Manage non-compliance issues through structured documentation
- Ensure employee adherence to standardised processes
- Streamline audit management and meet regulatory requirements

Explore the Unmatchable Features Pharmaceutical QMS Offers
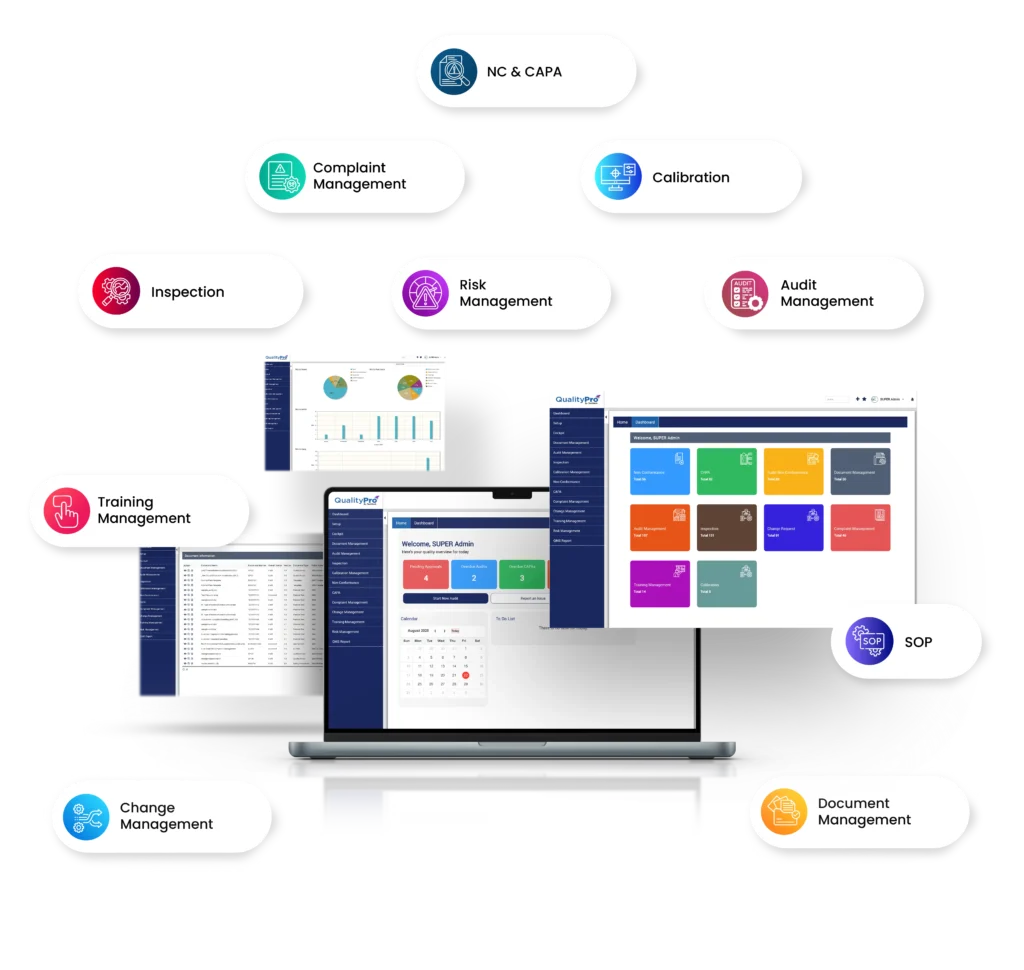
Efficiently manage Non-Conformance (NC) issues such as contamination, quality deviations, packaging defects, and compliance breaches. Identify root causes and apply Corrective And Preventive Actions (CAPA) to prevent recurrence…Read More
Track, manage, and resolve customer complaints related to drug side effects, inadequate packaging, expiry dates, and other product concerns, ensuring continuous improvement and customer satisfaction...Read More
Automate training processes, create schedules, and monitor employee knowledge on GMP compliance, safety protocols, pharmaceutical workflows, and quality management in pharmaceutical industry. Maintain accurate training records and evaluate employee competency...Read More
Establish a structured change control process to document, evaluate, review, and manage modifications in pharmaceutical quality management systems, ensuring regulatory compliance and operational efficiency...Read More
Create, store, and track pharma QMS documentation, including drug safety protocols, chemical specifications, regulatory records, and quality reports. Enable real-time collaboration for improved productivity...Read More
Identify and mitigate risks affecting product quality and patient safety. Implement proactive quality management systems in pharma to drive continuous process improvement and ensure regulatory compliance…Read More
Ensure seamless internal and external audits with centralised storage of critical compliance documents. Conduct timely regulatory, GMP, and drug quality audits to maintain industry standards...Read More
Digitally manage and standardise all Standard Operating Procedures — from formulation, compounding, and mixing to final packaging and release — ensuring each process follows approved, updated, and compliant instructions.
Plan, perform, and record in-process and final inspections for raw materials, intermediates, and finished products. Ensure adherence to GMP, FDA, and other regulatory quality standards through digital inspection checklists and real-time reporting.
Schedule, track, and maintain calibration of lab instruments, weighing balances, filling machines, and testing equipment to ensure precision, accuracy, and compliance with ISO, FDA, and other pharmaceutical regulations.
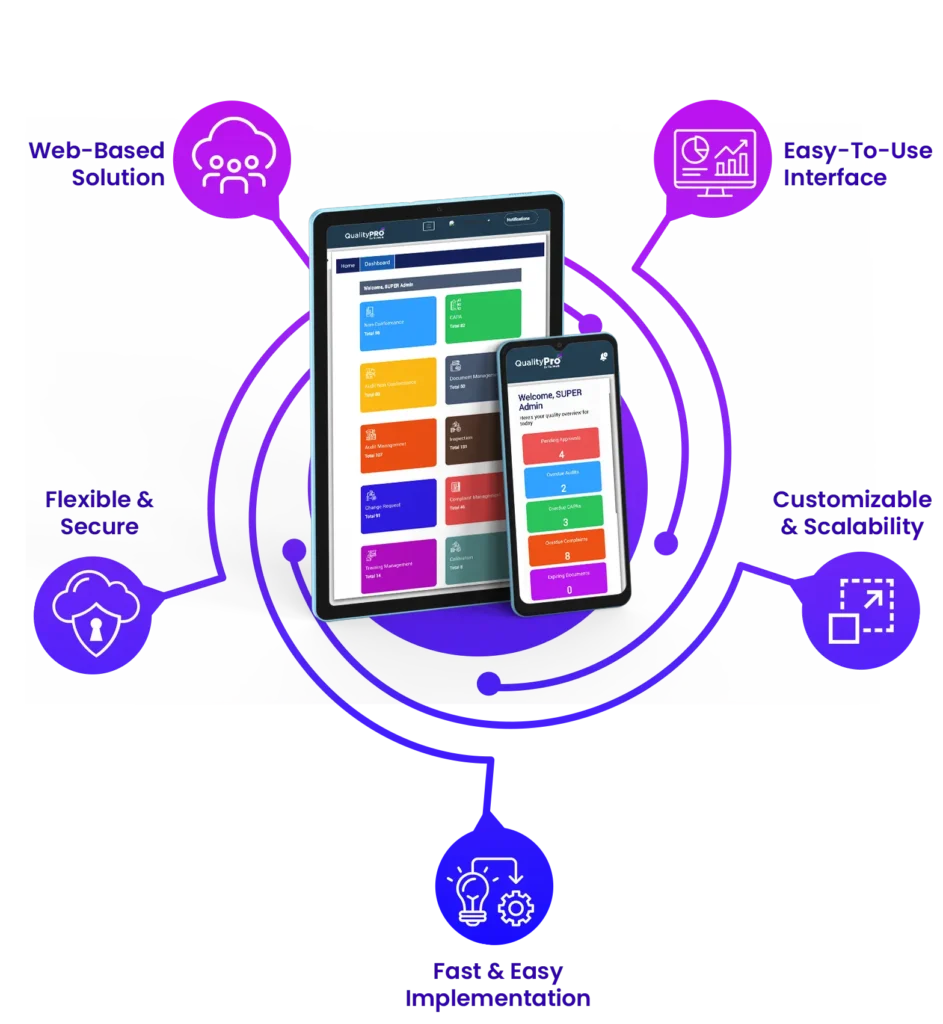
Discover the Advantages of QMS Software for Pharmaceutical Manufacturing
- Intuitive Pharma QMS for Plant Teams: QualityPro’s easy-to-use interface allows production, lab, and QA staff to navigate effortlessly, even without technical expertise. From raw material handling to final packaging, this pharmaceutical QMS ensures smooth, hands-on daily operations.
- Cloud-Based QMS for Real-Time Pharma Production: Track quality checks at every step from material inspection, formulation, and batch processing to final product release with secure, cloud-enabled access. This QMS supports fast deployment, collaborative workflows, and automated updates to maintain GMP, FDA, and ISO compliance.
- Tailored QMS for Pharma Requirements: Record batch-specific assay results, stability data, lab tests, and packaging checks with ease. QualityPro’s pharma QMS adapts to your specific processes and regulatory needs.
- Quick Setup with Flexible Implementation: Choose self-deployment or guided onboarding to match your plant’s workflow and team readiness. Get up and running quickly with minimal disruption to production.
- Grows with Your Operations: As you expand — introducing new formulations, production lines, or export batches — pharmaceutical quality management software scales alongside your operations, ensuring uniform quality and control across all locations.
- Scalable QMS for All Pharma Business Sizes: From small labs to growing pharma brands or multi-site manufacturers, this system fits any scale — from pilot batches to high-volume commercial production.
- Secure and Reliable for Critical Pharma Data: Protect sensitive records such as batch logs, lab results, vendor documentation, and compliance reports with enterprise-grade encryption, secure logins, and multi-tier backups. QualityPro’s QMS for pharma industry provides complete confidence in managing a highly regulated industry.
“Just one click, and you are one step closer to a transformation”
Know right away how QualityPro can transform your business
Frequently Asked Questions

A Pharmaceutical Quality Management System is a structured framework of processes, procedures, and responsibilities designed to ensure that pharmaceutical products consistently meet quality standards and regulatory requirements. It encompasses quality along all aspects of production, from raw material procurement to final product distribution, ensuring safety, efficacy, and compliance.
Pharmaceutical Quality Management Software is vital for:
- Ensuring consistent product quality and patient safety.
- Facilitating compliance with stringent regulatory standards.
- Streamlining processes to enhance operational efficiency.
- Identifying and mitigating risks associated with product development and manufacturing.
- Promoting continuous improvement through systematic monitoring and evaluation.
QualityPro Pharma QMS facilitates the identification, documentation, and analysis of non-conformances through its integrated NC & CAPA Management module. It enables organisations to determine root causes, implement corrective and preventive actions, and monitor their effectiveness, thereby fostering a culture of continuous improvement and compliance.
Absolutely. QualityPro QMS for pharma is designed to be scalable and flexible, accommodating the needs of pharmaceutical companies ranging from small enterprises to large multinational corporations. Its modular structure allows organisations to tailor the system to their specific requirements and expand functionality as needed.
Implementing QualityPro involves several key steps:
- Needs Assessment: Evaluating the organisation’s specific requirements and objectives.
- System Configuration: Customising QualityPro’s modules to align with organisational processes.
- Data Migration: Transferring existing quality data into the QualityPro system securely.
- Training: Providing comprehensive training to users for effective system utilisation.
- Validation: Ensuring the system operates as intended and complies with regulatory requirements.
- Go-Live: Officially launching the system within the organisation.
Post-implementation support is also provided to address any issues and ensure smooth operations.
About Us
QualityPro is an eQMS software by TecWork Global Business Solutions, specially tailored for industries such as Electronics, Manufacturing, Electric Vehicles, Automotive, Food and Beverages, Solar panels, Pipe, Steel and more. TecWork Global Business Solutions, the parent company, endeavours to converge cutting-edge technology with business excellence. At TecWork, we are dedicated to empowering businesses through innovative solutions that specialise in and seamlessly align with diverse business objectives such as fleet management software, field force management software, and others.

