Paperless Future: Digital Document Management for Food and Beverage Industry
If it’s not documented, it doesn’t exist!
The above proverb fits perfectly for the food and beverage industry. Documentation acts as a mirror for the food and beverage companies. It is a trustworthy source of information on everything happening inside and outside the industry, covering the entire process from material sourcing to final product.
Managing and securing documents are two super-important aspects for the food and beverage industry. Key records like recipes, ingredient specifications, safety checklists, SOPs, and BMRs are some of the documents that are important for this industry, and these documents play a big role in ensuring product quality, food safety, and compliance with strict regulations like FSSAI, FDA, and ISO 22000.
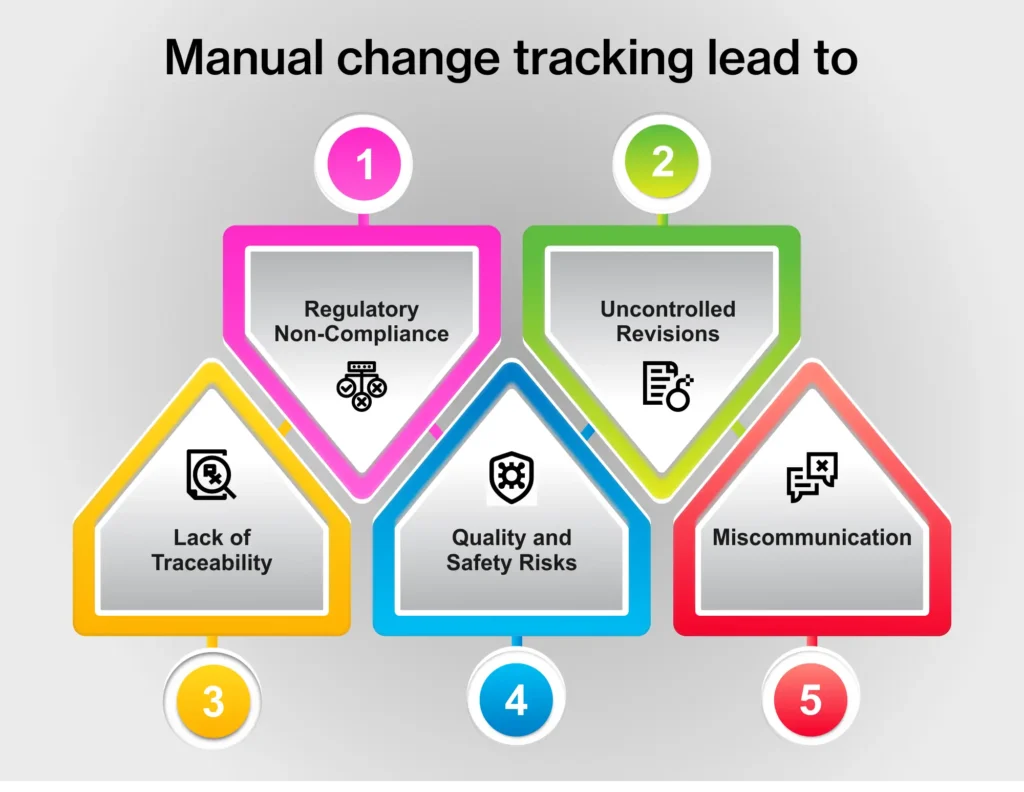
When food manufacturers rely on paper-based systems, they risk more than just lost time. Misplaced recipes, improper HACCP plans, or erroneous cleaning and temperature logs can lead to compliance failures, product recalls, or worse—falling into the wrong hands.
Imagine a competitor gaining access to your proprietary formulations or critical food safety procedures. That’s not just a data loss—it’s a business risk you can’t afford. Undebated, paper-based processes are also inefficient and money-draining. Stats confirm it too.
Employees spend up to 30% of their time searching for paper documents.
— Source: IDC (International Data Corporation)
It takes an average of 18 minutes to locate a paper document, and 50% of documents are misfiled or lost.
— Source: AIIM (Association for Intelligent Information Management)
As food and beverage businesses grow and regulatory requirements rise, paper records are becoming outdated. They’re time-consuming to manage, prone to errors, and difficult to organise across files and folders. Because of this, food and beverage companies are now putting Document Management Systems (DMS) into place.
With food and beverage document management software, important papers like allergen information, traceability reports, and nutrition labels are stored in one place. Teams can easily work together, monitor changes, always access the latest version, and stay compliant and audit ready.
By moving critical documentation—like allergen declarations, traceability reports, or nutritional labeling sheets—to a centralised digital system, teams can collaborate in real time, automate approvals, instantly access the right version, and maintain full traceability—helping them stay compliant, efficient, and always audit-ready.
What is a Document Management System?
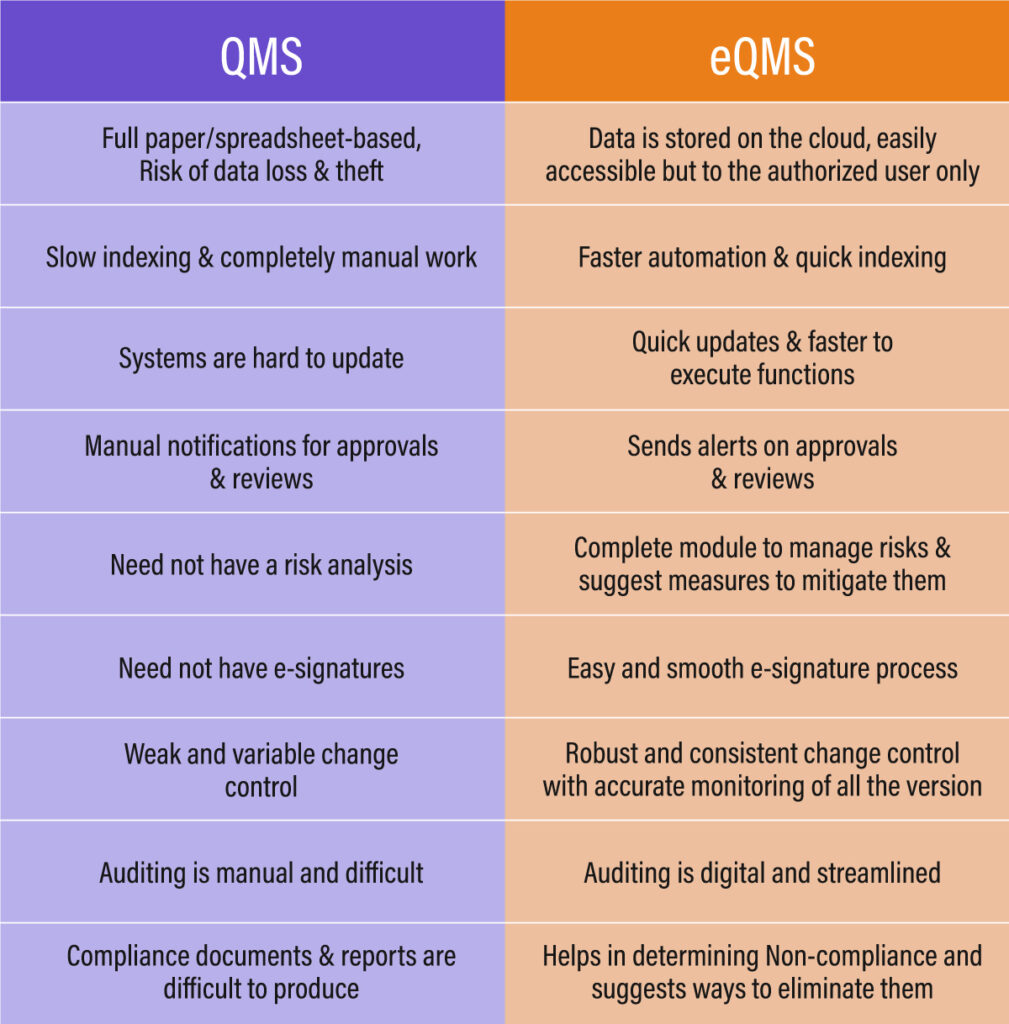
A document management system is defined as a systematic set of procedures and policies related to creating, capturing, storing, and tracking documents efficiently within an organisation. However, manual management of such a large volume of documents poses inherent risks and inefficiencies.
Recognising this, a crucial need for an automated document management software arises. It eases the task of document management by providing a centralised, organised, and secured platform, enhancing the efficiency of tasks related to documents.
How QMS Software Simplifies Food and Beverage Document Management
An advanced Quality Management System (QMS) with an integrated Document Management System (DMS) can significantly enhance document handling in the food and beverage industry—eliminating time- and cost-intensive paper-based processes, improving data security, and ensuring more structured, accessible documentation.
A Food and Beverage Quality Management System helps store, organise, and control access to essential files—like HACCP plans, product sampling reports, labeling protocols, and SOPs—within a unified, secure platform. An advanced QMS simplifies and transforms food and beverage document management by –
Automating the Process of Document Management-
The software automates the complete document lifecycle, right from creating to controlling, managing, organising, and retrieving when required. Moreover, it also manages multiple versions of documents separately, ensuring that only the Active version is being accessed by everyone.
Ensuring Timely Updates of Documents–
The software helps users to ensure that all the documents are up to date by providing alerts for expiry or whenever a document is due for revision/updates. It also sends notifications for release or pending approvals of documents.
Providing Easy Access to Crucial Documents-
By organising all the documents at a centralised location, categorising them based on file type, data type, and priority level, and allowing users to search documents via metatags, a food and beverage document management software facilitates easy and quick access anytime, anywhere.
Ensuring Safety through Access Control–
By enforcing access controls over the documents via passwords, a food document control software prevents the risks of data theft or lost documents, ensuring tightened safety. It also tracks all the activities performed on the documents, maintaining an audit trail of who and when viewed and modified the document.
Allows Effective Collaboration-
A robust document management software enables such a capability that allows multiple people to collaborate and work on a common document without altering each other’s work.
QualityPro – An Ideal QMS for Managing Documents
QualityPro QMS is a cloud-based quality management software with an extensive document management system. This food and beverage document management software covers the whole document lifecycle within an organisation, from content creation and publication to archiving and, finally, secure retention and disposal of content.
Here’s How Food and Beverage Document Management Software Supports Documentation Needs:
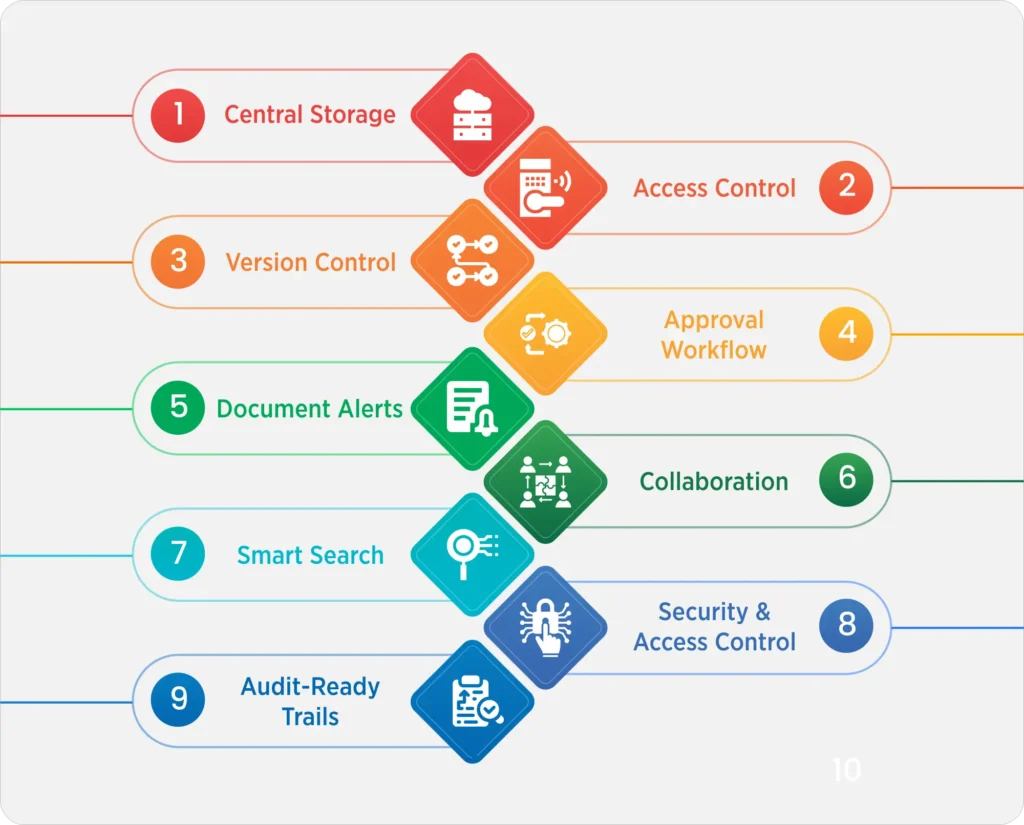
Central Storage:
The document management module of QualityPro allows you to store all critical documents in the cloud and access them from anywhere and everywhere.
Access Control:
Access control system ensures that a document and/or its version can only be accessed by the team(s) to whom access control has been provided.
Version Control:
Document Version Control is a very important part of the document management in food and beverage industry that allows you to record and track minor and major changes to a document, ensuring that only the right version of the right document falls into the right hands at any given time.
Approval Workflow:
One of the best parts of the DMS software for food and beverage is that you can set up a multi-level approval system, thereby eliminating wastage of time on document approvals and corrections. The supersede feature allows users to skip the approval of a lower hierarchy user and get direct approvals to save time.
Document Alerts:
Document management in food and beverage allows you to set alerts for various documents that could include version changes, approval alerts, document expiration alerts, and more.
Collaboration:
The food industry document management software allows cross-functional and cross-location teams to collaborate and create/work on the latest version of a document, together, in real-time.
Smart Search:
The smart search feature of the DMS system allows users to find documents really quickly by using search tags.
Security & Access Control:
Document management system for food and beverages Industry allows you to lock access to files with password protection.
Audit-Ready Trails:
The system can help you do the audit, maintain audit trails, as well as inspection checklists. It also helps maintain complete logs to support inspections by FSSAI, ISO, BRCGS, or for customer audits.
Time to Go Digital
With help from QualityPro, make your food and beverage business documentation easier, more effective, efficient, and economical in every way. Come, let’s turn paper trails into digital confidence and take advantage of this powerful food and beverage document management software today!
Think Document Handling — Schedule a demo of QualityPro’s Document Management System today.